Autorship: Beatriz Moraes Murer e Silvia de Melo Futada
This section provides information on one of the greatest riches we have: biodiversity. What is biodiversity? What is its importance and its relation to so-called ecosystem services? What do we know about it? What are possible approaches to understanding it?
Definitions, examples and background to aspects of landscape, information on terrestrial and coastal ecosystems, caves and all Brazilian biomes. As well as considerations on agrobiodiversity offered a member of the Public Prosecution Service of the Federal District and founding partner of ISA, the late Juliana Santilli.
What is it?
The term 'biodiversity' was coined from the term 'biological diversity' but transcends its original meaning. In the early 1980s, 'biological diversity' was synonymous with species richness; in 1982, the term acquired the sense of genetic diversity and species richness; and finally, in 1986, with the contraction of expression, it expanded to cover, in addition to genetic diversity and species diversity, ecological diversity.
In 1986, at the 1st National Forum on BioDiversity, organized by the US National Research Council (NRC), E.O. Wilson replaced the term “Biological Diversity” with “Biodiversity”, and the ensuing publication1 contributed to the rapid adoption of the term by scientists almost immediately2, as well as by environmentalists, political leaders and citizens worldwide.
In 1992, the Convention on Biological Diversity (CBD), popularly known as the Biodiversity Convention, defined in its second article the term 'biological diversity' as the 'variability among living organisms from all sources including, inter alia, terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are part; this includes diversity within species, between species and of ecosystems’. Despite the controversies surrounding this definition, the CBD consolidated a way of viewing biodiversity and contributed to placing this on the political agenda of the 196 countries who have ratified the Convention.
Biodiversity as understood in the CBD, therefore encompasses genetic variability and the diversity of species, ecosystems and landscapes. In addition, it also covers the relationships among these components of biodiversity, i.e. their function and structure.
Thus, it is usual to consider2 intraspecific diversity (within the same species) covering all the variation between individuals of a population, as well as between spatially distinct populations of the same species. In practice, this diversity has been considered as equivalent to genetic diversity, although it may include morphological, behavioural or other forms of diversity, without being strictly based on the genetic basis of such differences.
Interspecific diversity (between species), in turn, corresponds to what is called species diversity, that is, the variety of species existing in a given environment or defined region.
For its part, the diversity of ecosystems has been treated as related to the diversity of physiognomies of vegetation, landscape or biomes. However, although ecosystems are essentially functional systems characterized by their dynamics, using dynamics as a basis for evaluating, inventorying or monitoring the diversity of ecosystems is impractical, though not impossible.
See the table below for further definitions of biodiversity.
Definitions of biodiversity
In the early 1990s, several researchers were interviewed by David Takacs, who, among other outputs, compiled a series of definitions of the term "biodiversity"3. In these definitions it is possible to identify at least two major currents: the one that focuses on species diversity and one that considers biological processes, in one form or another, as part of biodiversity. Here are some examples:
- "The variety of genetically distinct populations and species of plants, animals and microorganisms with which man shares the earth and the variety of ecosystems of which they are integral parts." Paul Ehrlich, 1992.
- "Biodiversity is the total number of genetic lineages on earth." Thomas Eisner, 1992.
- "Biodiversity is the sum of the earth's species including all their interactions and variations with their biotic and abiotic environment in space and time." Terry Erwin, 1991
- "The dimension of difference in the multiple levels of biological organization, considering all the different entities and all the different processes ". Donald Falk, 1992.
- "The sum of genes, populations, species and the set of interactions they reveal." Daniel Janzen, 1992.
- "Variation, variability or variety of living organisms, including intraspecific variation and levels of communities, ecosystems and landscapes". KC Kim, 1992.
- " Diversity of all levels of organization". Thomas Lovejoy, 1992
- "The total biotic diversity indicated as the number of species and the genetic diversity they cover ". SJ McNaughton, 1992.
- "Variety of life and its processes". Reed Noss, 1992.
- "Diversity of species that exist in a given ecosystem". David Pimentel, 1992.
- "The sum total of plants, animals, fungi and microorganisms in the world, including their genetic diversity and the involvement of all in communities and ecosystems." Peter Raven, 1992.
- "It is life in all its dimensions, wealth and manifestations, not only at the level of individuals and species, but also at the level of aggregations, communities, etc." Michael Soulé, 1992.
- "Variety of life across all levels of organization, from gene diversity to populations, species diversity, which must be viewed as the pivotal unit of classification, to the diversity of ecosystems." Edward O. Wilson, 1992.
Biological systems are not limited to the mere presence of organisms; their functioning depends on organisms and the interactions between these. For this reason, it has been argued4 that biodiversity has three fundamental components by which it can be measured: composition, structure and function. Composition refers to those elements that constitute the biological unit; structure, to how these organize themselves physically; and function is related to the fact of ecological or evolutionary processes being maintained or produced by the biological unit in question. Thus, biodiversity encompasses the processes that generate and maintain species, genetic variability, diversity of populations and communities, the multiplicity of ecosystems and landscapes, as well as all their relationships with the physical environment and with each other.
As well as species numbers, other aspects of biodiversity may be represented by the number of trophic levels present, the number of guilds, the variety of life cycles and the diversity of biological resources, and these should be taken into account when ecological and environmental characterization is sought. Some species may play an important role in overall species richness, such as pollinators, mutualistic symbionts, population-regulating pathogens, and biological control agents with effects on local biodiversity.
Thus, the argument of scientists and researchers that the conservation of isolated genes, species and communities is not enough to conserve all the desired biological phenomena makes sense, since some result, for example, from unique interactions between certain components of the systems.
Why is genetic diversity important?
In the early nineteenth century, potatoes were one of the products most cultivated in continental Europe and the British Isles. In Ireland, potatoes became the basis of the economy and diet, especially for peasants, who ate nothing but potatoes, considered a nutritionally well-balanced food, during the long winters. However, in 1845, a disease attacked the harvest, destroying it completely and causing unprecedented hunger.
This potato blight in Ireland illustrates a constant dilemma of agriculture, which in turn brings us to the question of genetic variability. To produce the 'best plant' which will provide maximum yield, farmers and scientists will cross and select plants over generations until the right mix of specific characteristics is achieved. Then they develop the entire seed stock from this improved form; that is, all the plants have a single parent, they are genetically uniform. Genetic variability is exchanged for the perfect item of food. However, the lack of genetic diversity makes the single variety very susceptible to disease: if a fungus, virus or bacterium attacks the plants successfully, it can devastate the entire crop, since the plants are all genetically the same and have the same levels of defence and adaptation.
The Irish grew their potatoes from a single 'best plant'. With the arrival of the disease, caused by a fungus, they lost not only the entire 1845 crop, but also the possibility of a subsequent recovery, through the planting of resistant varieties. In natural populations, on the other hand, the genetic diversity of individuals ensures that some will be immune to the disease and that part of the crop will survive. These survivors will give rise to the following year’s plants which will consequently be resistant to that disease.
Similar stories can be told for many of the major agricultural products, and crop protection resides in most cases in the genetic diversity found in wild varieties. In addition to the fact that these wild varieties are found only in their natural environment, often in specially protected spaces, they need to continue living in these environments in order to remain as potential genetic stock, a factor preventing future diseases. It is the fact of continuing to live in its natural environment that allows the organism to change over time and to remain adapted to the changing environmental conditions.
Weaknesses such as this do not only occur in relation to natural pests, such as potatoes and fungi, but also in relation to climate change. Imagine a plant adapted to the specific conditions of its environment, such as a rainfall regime and a certain temperature range, which is suddenly exposed to other conditions: the temperature rises and rains become scarce, causing prolonged droughts. Our hypothetical species will suffer greatly, and some less resilient individuals will certainly die. Nevertheless, thanks to the genetic variability among its individuals, there are some that are more resistant and better able to survive in these new environmental conditions. These will reproduce and generate new plants, adapted to the new conditions, allowing the species to survive in that place. In the case of climate change, this diversity is essential and can mean the extinction or not of a species.
What does the Queen of Hearts have to do with biodiversity?
When Alice, lost in Wonderland, meets the Queen of Hearts and wishes to ask her some questions, she realizes that to stay by her side, she would have to run non-stop. But no matter how hard she ran, she stayed in the same place, and that was the only possible way to talk to the queen ... Genetic diversity has a similar role, it allows living beings to continue "running" to stay in the same place and survive. That is, since the environment in which we live is dynamic, living beings need to constantly change to remain adapted to the conditions of the environment and thus survive.
The importance of biodiversity is not restricted to the intrinsic value of species, just looking around and inwards makes us realize that much of what we need and enjoy comes from nature: the wood of the table on which we are working; the paper on which we write; our food; the clothes we wear; the fun we have in parks, waterfalls, beaches; and many other things. In the same way, there are other processes, also essential for our survival, but which are provided by nature and not so easily perceived: atmospheric regulation, nutrient cycling, soil conservation, water quality, photosynthesis, decomposition, etc. They are goods and services obtained directly and indirectly from ecosystems, which provide the conditions for the maintenance of our species and are known as ecosystem services.
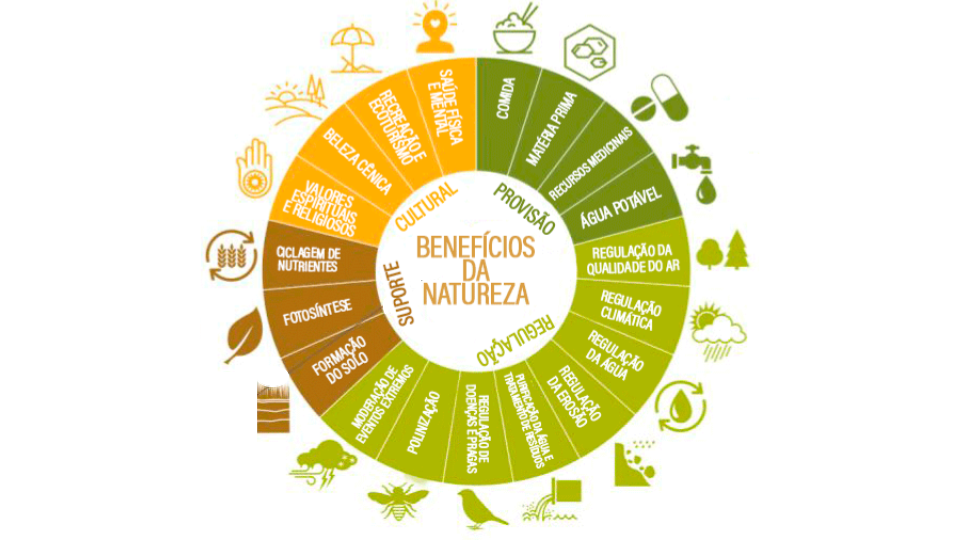
These goods and services are classified into four groups, according to the MEA (Millenium Ecosystem Assessment5): provision, regulation, support and cultural (Figure). This is the classification most used, although some changes have been proposed. Provisioning services include the products obtained from ecosystems and which are supplied directly to society, such as food and natural fibres, fuel, water, genetic resources, among others. The Regulatory services provide the benefits derived by society from the natural regulation of ecosystem processes, such as climate regulation; maintenance of air quality and pollution control by regulating the composition of atmospheric gases; regulation of water flows (hydrological cycle) and flood control, avoiding floods and contributing to the recharge of aquifers; erosion control; water purification; reduction of pests and diseases through biological control, regulation of natural damage and pollination of agricultural and wild plants.
Support services allow the necessary conditions for other services to be made available to society. Thus, benefits are generally indirect and manifested over the long term, such as the formation and maintenance of soil fertility, oxygen production, nutrient cycling and primary production. In addition, they include biodiversity (genetic and species), found in natural environments that support ecosystem functioning, giving them resilience to external changes. In other services, benefits are direct and usually occur over shorter terms. For example, society does not directly use the service of soil formation, although changes in this indirectly affect welfare, because they alter the flow of the production service. Cultural services, correspond to the non-material benefits of ecosystems, which contribute to the well-being of society, such as spiritual and cultural enrichment, cognitive development, reflection on natural processes, leisure opportunities, ecotourism and recreation.
In 2018 the Summary for Decision-makers6 of the 1st Brazilian Assessment of Biodiversity and Ecosystem Services was published, prepared by the Brazilian Platform for Biodiversity and Ecosystem Services (BPBES), supported by the BIOTA-FAPESP Programme. The researchers concluded that, although biodiversity and ecosystem services are perceived in Brazil as obstacles to economic development, they are fundamental elements for coping with socioeconomic and environmental crises. This is because they bring new development opportunities, for example in the areas of tourism, cosmetics, pharmaceuticals and food. The Brazilian Platform operates under the International Platform, created in 2012 by more than 100 governments as a mechanism to provide scientific information in response to requests from decision-makers under the umbrella of four United Nations bodies: UNEP, FAO, UNDP and UNESCO, and managed by UNEP. IPBES brings together scientists and other knowledge holders around the world to analyse and evaluate relevant scientific data and techniques produced worldwide, in order to understand biodiversity and ecosystem services.
Thus, the ecosystem services approach came from the perspective of bringing together nature and the economic approach, in order to value the goods and services provided to humans by the environment. The approach has firmed up and emerged classifications of goods and services have emerged, but the important message of this approach is that our health, food and safety depend directly and indirectly on biodiversity. From drugs to food production, biodiversity is essential to human well-being. Understanding this relationship and interdependence is important for decision making in the context of environmental pressures and challenges ambientais, which put ecosystem functions and services at risk and therefore also impact human life. Read more about this in the statement by Nurit Bensusan.
What do we know about biodiversity??
Biodiversity, therefore, is an important matter which directly and indirectly influences human life and involves genetic, species, ecosystem and landscape variability. Despite this, little is effectively known about biodiversity.
How many species are there on Earth? How many have there been? Such questions are still puzzling, since estimates often depart from highly controversial assumptions. In fact, faced with the question 'what we know about biodiversity?', a better answer would be 'we do not know how much what we know really represents'.
If we deal solely with the number of species on the planet, there are estimates ranging from around two million to six billion. Since the 1980s, tropical rainforest beetles have raised expectations. Even today, access to previously unseen areas such as the poles and the depths of the sea, has also caused estimates to rise.
Most of the species that have been described by humans, that is, identified and named, are made up of insects. Of the approximately 1.9 million living species already described up to 2018, about half are insects, including 300,000 beetles, and only about five thousand are mammals like us.
In tropical countries with great diversity, such as Brazil, there is also the question of the lack of data, not only due to the richness (number of species) and abundance (number of individuals per species), but also due to the reduced number of specialists and to regions of difficult access. It is estimated that the Brazilian biota corresponds to approximately 13% of the world biota, and greater knowledge of these species is fundamental to succeeding in the conservation and sustainable use of our rich heritage. Between the years 1978 and 1995 alone, in Brazil 7,302 species of animals were described, 69% of which were insects (in fact, this estimate is for a taxonomic group called metazoan, which includes most of these animals, excluding only a small group little known except to specialists)7 and 8.
The table below shows the number of known species recorded in Brazil and in the world9. he grouping has been done with a certain taxonomic freedom to provide a better understanding by non-expert readers, but the original tables with their details can be directly accessed in the publications.
| Group | Species in Brazil | Species in the world |
|---|---|---|
| Virus | 310 - 410 | 3.600 |
| Bacteria | 800 - 900 | 4.300 |
| Fungi | 13.090 - 14.510 | 98.998 |
| Protozoa | 7.650 - 10.320 | 76.100 - 81.300 |
| Plants | 43.020 - 49.520 | 310.129 |
| Total Animals | 103.780 - 136.990 | 1.424.153 |
| *Invertebrates | 96.660 - 129.840 | 1.359.365 |
| *Fish | 3.420 | 31.153 |
| *Amphibians | 775 | 6.515 |
| *Reptiles | 633 | 8.734 |
| *Birds | 1.696 | 9.990 |
| *Mammals | 541 | 5.487 |
| GRAND TOTAL | 168.640 - 212.650 | 1.800.000 - 2.000.000 |
It is believed that many of the species that exist and have not been described are small, poorly coloured species with restricted geographic distributions and threatened with extinction, which makes them difficult to identify and catalogue. Moreover, as well as knowing which species they are, we need to understand where they are, their abundance and their functional attributes and functions, as well as their ecological role, and to develop conservation and management policies.
Ecological Scraps
LANDSCAPE
The first reference to the word "landscape" appears around 1000 BC, in the "Book of Psalms," where it has a visual and aesthetic connotation; this was followed by literature and the arts in general, mainly by painting in the second half of the 18th century. Today the landscape is defined by the dictionary, as "an extent of territory taken in by a single glance". The word "landscape"10 therefore permeates the arts, geography, geology, architecture and ecology, and thus has different connotations depending on the context and the person using it.
Landscape ecology is a branch of knowledge within ecology, marked by the integration of two main approaches: the geographical, which stresses the study of human influence on the landscape and land management; and the ecological, which stresses the importance of the spatial context on ecological processes and the importance of these relations to biological conservation. Landscape ecology is therefore a combination of the spatial analysis of geography with the functional analysis of ecology.
This approach offers the integration needed for practical application, since it proposes to view environments as anthropized mosaics, on the scale at which humans are modifying their environment. In the "geographical approach", we try to understand the structural, and therefore functional, changes brought about by humans in the mosaic as a whole, explicitly incorporating all the complexity of the spatial interrelations of its components, both natural and cultural. In the "ecological approach", we have the right scale to respond to major environmental problems, both those related to habitat fragmentation and those arising from unsuitable use of soil and water.
To make land use compatible with environmental, social and economic sustainability, it is necessary to plan the utilization and conservation of the landscape as a whole. For example, protection of only a fragment of vegetation or a section of the river is insufficient if there is degradation of the area surrounding the fragment or to the river’s headwaters. Humans are the root of much environmental damage, but they are also part of the solution. The conflict lies not in humanity as such, but with predatory development models that seek to subjugate systemic processes and turn them into products. In dealing with the landscape as a whole, taking the spatial interactions between cultural and natural areas into account, and thus including us in its system of analysis, landscape ecology offers a suitable perspective for proposing solutions to environmental problems.
Fragmentation
Fragmentation is the name given to the process by which, following disturbance, a large expanse of habitat is subdivided into smaller fragments, which have smaller total area than the initial habitat and are isolated from each other by a habitat matrix different from the original.11. This process is a response to the removal of native vegetation and occupation of the territory by production activities and habitation (urban settlements). It is one of the main causes of biodiversity loss globally.
Habitat fragmentation introduces a break in continuity of the distribution of the original vegetation, reduces the habitat available to organisms and creates edges to a landscape until then continuous. This process may imply loss of biodiversity and changes in the distribution and abundance of organisms.
Fragments may differ in shape, size, microclimate, light regime, soils, level of isolation and type of land use. In this way a continuous landscape, once fragmentation begins, becomes a landscape containing previously non-existent elements. The characteristics of these elements and the way they interact promote biotic and abiotic responses that alter the composition and structure of the community, as well as the spatial pattern of the landscape.
Drastic changes in landscape geometry and attributes alter the microclimate of the habitat, exposing organisms to sunshine, winds and desiccation12, changing the composition and structure of the community, since some species are extremely dependent on optimal abiotic conditions for germination, growth, establishment, flowering and pollination. Fragmentation also influences interactions between plants and animals, consequently affecting the population density of pollinators and dispersers and the demography and recruitment of plants13.
In a fragmented landscape three components are recognized: the matrix, the most extensive component of the landscape, highly connected and which controls regional dynamics14 – for example, a soybean, pine or pasture plantation, or even the urban footprint in the case of cities; fragments, emnants of the original habitat, spatially reorganized into smaller areas and smaller total area, which show a degree of isolation between them; and corridors, areas that are different from the matrix and connect the fragments15.
The composition and permeability of the matrix are variables that alter the connectivity of the landscape, connectivity being the capacity of the landscape to facilitate biological flows, that is, the spatial continuity of the original habitat14. Connectivity depends on the proportion of the landscape the fragments occupy, a feature directly related to the size of the fragments and the degree of isolation between them, whether barriers are spatial or ecological. Larger fragments with greater habitat diversity would be better able to maintain ecological processes and a greater number of viable species and populations. This is also closely related to the existence of corridors16, which, in addition to their ability to connect fragments, facilitating the survival of organisms during their flow between areas, may present the necessary conditions for temporary or permanent habitat for some populations.
Therefore, in a landscape whose matrix has low permeability, the mobility of organisms and their consequent dispersion and colonization of new fragments is undermined. This can be illustrated by a remnant of forest isolated in an urban environment with the probability of traffic running over the fauna. A more permeable matrix would be an environment whose changes have not been so drastic and are close to the original environment.
Coastal Ecosystems
Bearing in mind Brazil’s extensive coastline, its coastal and marine ecosystems correspond to an enormous and biodiverse ecological transect. They occupy approximately 4.5 million km2 of territory under Brazilian jurisdiction. This represents more than a geopolitical concept; it includes and encompasses large extensions of landscape and diverse biological and human life.
Along the coast and seas of Brazil, where nearly a quarter of the Brazilian population lives - about 50 million inhabitants – there are fishing communities, extractive gatherers, artisanal fishers and other coastal peoples17 e 18, among which are approximately 800,000 fisher men and women, out of two million people responsible for 55% of the national fishery production18. These traditional inhabitants of our coast look after indigenous, African and Portuguese cultural heritage, to be seen in the varied techniques of capture, such as timbó, espinhel, tarrafa and fish processing, in the houses, in the music (the viola, the tambourine, the box and the accordion) and festivals (such as fandango), marked by the meeting of these cultures, which populate the universe of the caiçara, the jangadeiro, the crab collector and other coastal peoples.
The Coastal Zone is part of the national heritage, as stipulated by article 225 of the 1988 Federal Constitution of Brazil and extends for more than 8,500 km from north to the south of the country, where the land faces the Atlantic Ocean. The terrestrial area of the Coastal Zone is variable and covers 17 States and more than 400 Municipalities. It also includes the territorial sea, corresponding to the area within 12 nautical miles of the coast, thereby extending the area to 514,000 km2. The Marine Zone begins in the Coastal Zone and includes the continental shelf and the Exclusive Economic Zone (EEZ), which in Brazil’s case corresponds to 200 nautical miles from the coast19,20,21.
The coastal zone comprises regions of ecological transition with the important function of promoting connection and genetic exchanges between terrestrial and marine ecosystems, as well as being a barrier against flooding, saline intrusion and erosion19. It consists of ecosystems consisting of restingas, dunes, sandy banks, estuaries and deltas, coastal lagoons, mangroves, marshes, rocky bottoms and coasts, coral and sandstone reefs, limestone algae banks, sandy platforms and oceanic islands (such as Fernando de Noronha resulting from volcanic activity and which resurfaced separated from the continent); or of low lying sedimentary relief, such as Ilha Comprida on the coast of São Paulo, which is a restinga segment cut off by the sea, among others19.
Such complexity and diversity of environments are extremely important to sustaining all biological marine life and high productivity, thanks to the great concentration of nutrients that arrive transported by rivers and underwater sources and by the supply of nutrients by its very varied ecosystems. Due to the wide diversity and importance of such integration between land and sea systems, the coastal zone requires greater attention to its environmental integrity and balance since it serves a variety of situations, from intensive exploitation of its natural resources to the siting of a complex set of industries, ports, leisure and tourism facilities, and human settlement itself.
Marine biodiversity is still little understood by science. This environment, being further from the coast and due to its great depths, currents and storms, is less susceptible to direct human contact. However, it is affected by overfishing and intense oil extraction, as well as being the repository for the most varied wastes and discharges of chemical pollutants from the continents.
Conservation areas to protect this environmental mosaic that includes the ecosystems of the Marine and Coastal Zone are still few. The need to extend their protection also lies in the internationally recognized importance of marine ecosystems in mitigating the effects of global climate change, with their ability to absorb large amounts of carbon dioxide (CO2) from the atmosphere.
In 2017, protected areas covered 13.2% of the marine environment under national jurisdiction (up to 200 nautical miles from the coast), 0.25% of the marine environment outside national jurisdiction and 5.3% of the world's oceans. There are 4.977 km2 of full protection areas and 50,739 km2 of sustainable use areas, representing 1.57% of the total of these biomes16.
Brazil has signed and ratified (in 1988) the United Nations Convention on the Law of the Sea (UNCLOS), in force since 1994, which gives states exclusive rights over the use and exploitation of resources and their ‘territorial sea’. Under this agreement, countries agreed to establish further measures for the management and conservation of living resources in their EEZs. However, there is great concern about human pressures on this environment, which are increasing with the expansion of oil production in the pre-salt layers and of port infrastructure and the lack of more stringent sanctions for the inappropriate disposal of domestic and industrial waste. In addition, tourism, f not well planned, can exert strong pressure on the resources and functions of the marine and coastal ecosystem, together with shrimp farming (mostly exotic), which has also caused socio-environmental conflicts in this environment.
Attracted in part by the richness and great concentration of nutrients in the marine and coastal zone, shrimp farming, however, in addition to environmental damage, often causes traditional and local populations to lose their territories. Globally, about half the loss of mangroves can be attributed to the implantation of shrimp farming22. Overfishing also figures as a factor of intense pressure: in the medium term it reduces food production, impacts the functioning of ecosystems and reduces biodiversity. Research shows that marine fish stocks have declined worldwide from 90 per cent in 1974 to 68.6 per cent in 2013. With effective management and sufficient resources, marine protected areas are important mechanisms to safeguard world-wide scale.
Caves
Diversity also includes variety of geological formations, for example in the case of speleological formations, known as caves. Brazilian speleological heritage is extremely rich and enchanting, with great diversity in its associated geomorphology and biota. According to the database of the National Centre for Research and Conservation of Caves23 (CECAV) a body under the Chico Mendes Institute for Biodiversity Conservation (ICMBio), in December 2018 Brazil had 18,012 caves recorded on the National Cadastre of Speleological Information (CANIE). However, we know that this figure is still an underestimation and that this number represents only the caves already prospected and with published data, which have been systematized, geo-referenced and analysed by the CECAV.
The cave environment presents very special characteristics when compared to the above-ground environment. Among these are the permanent absence of light and the tendency for stability of environmental conditions, such as temperature and humidity24 and the great dependence on an external source of food.
The absence of light inside caves makes it impossible to maintain photosynthesising organisms, such as plants or algae. Thus, transfer of such resources to the interior of caves is carried out by water courses, temporary or perennial; water that percolates through openings in ceilings and walls; and remains of dead animals that use the caves as shelters or accidentally enter them. A third and very important food source for cave fauna is guano, rich organic material originating mainly from the faeces of cave-dwelling bats. In tropical caves, especially those permanently dry, bat guano is the most common source of organic material, forming the trophic base for the structure of invertebrate fauna communities.25
The low availability of resources in the caves acts as a factor limiting the establishment of numerous species and as selective pressure on the emergence of adaptations to the organisms that live in them. Cave species, therefore, present morphological, physiological and behavioural adaptations, usually related to the physical conditions of the habitat and the scarce availability of resources24. Characteristics of cave-dwelling animals can include small or absent eyes, little needed in dimly lit environments, but other well-developed sensory organs, such as antennas, or the absence of showy skin colourings.
According to CECAV23, in December 2018 there were 4,420 caves recorded in the Legal Amazon region. They can all be found on our interactive map. The Guy Collet Abyss cave, located in the municipality of Barcelos (Amazonas), an area of the municipality within the Serra do Aracá State Park, is estimated to be the deepest cave in Brazil, reaching a depth of 670 metres26.
The conservation of such sensitive and differentiated ecological systems is important for the conservation of rare minerals or unique geological formations and for activities such as leisure (recreational, sports and contemplative practices); strategic storage of water, with the charging and recharging of aquifers; as a source of information on geological processes, making it possible to investigate the origin, formation and successive transformations of the local rocks and the former paleoclimate of the region; as a provider of information of past life in its fossil and archaeological sites, allowing the possibility of identifying, cataloguing and researching fossils of animal and plant species, as well as the cultural study of past peoples whose documents, monuments and objects provide important records of the lived habits of a particular society; as a refuge for conserving habitats of endemic and endangered species; and as an environment for cultural and social events.
Biome
One of the possible ways of viewing landscapes is through sections known as biomes, a concept that is the fruit of wide discussion in science. A biome corresponds to an extensive area of geographic space, of up to a million square kilometres or more, with uniform characteristics with respect to a defined macroclimate, a particular phytophysiognomy or plant formation, fauna and other associated living organisms, and other environmental conditions, such as altitude, soil, floods, fire and salinity27. Such characteristics confer a particular structure and functionality, an ecology of its own. It is important to distinguish biomes from morphoclimatic domains. These concepts are not synonymous, since domains mainly relate to climatic, geological and relief characteristics. There is also the concept of phytophysiognomies, whose definition is related mainly to the composition of the vegetation of the place. Biomes usually consist of a complex of phytophysiognomies and formations, representing an ecologically related gradient. On the other hand, in a single domain you can find different biomes. To go deeper into the evolution of related academic concepts, read the article by Leopoldo Coutinho here.
There are six biomes in Brazil, according to the Brazilian Institute of Geography and Statistics (IBGE) and the Ministry of the Environment (MMA): Amazonia, Caatinga (semi-arid), Cerrado (tropical savanna), Atlantic Forest, Pampa and Pantanal (semi-humid). You can locate all these biomes and their relationships to conservation areas on our map mapa.
Brazilian vegetation classifications
See other classification models in the Brazilian Vegetation Technical Manual, by28, clicking here.
Amazonia
Son of the Forest
Son of the forest, water and wood
go in the light of my eyes,
and explain my way of loving the stars
and of bearing hope on my shoulders.
An unjust gash, mud on wood,
the strong water of childhood arrives and washes.
I grew up in the midst of wood,
the logs soaked, green firewood,
my mother complained of the smoke.
In fact, on opening my eyes I saw wood,
the beautiful itaúba timbers
of my grandfather's house in Bom Socorro,
where my father was born
and where I too was born.
I was the last to see the house still standing,
still in one piece the uprights were leaning,
abode of bats and termites.
Finally collapsed by the waters of many floods,
the house drowned
in a silence of slime, leaves, tiles.
But the house only died for good
when the uprights of my father’s memory caved in,
in this summer of his ninety years. - Thiago de Mello29
The Amazon Biome is the largest Brazilian biome, occupying almost 50% of the national territory. It is home to the largest tropical forest in the world and contains the richest biodiversity and the biggest hydrographic basin on the planet. It encompasses five states of the federation in their entirety (Acre, Amapá, Amazonas, Pará and Roraima), one almost entirely (Rondônia: 98.8%), more than half (54%) of Mato Grosso and 9% of Tocantins. The Amazon biome is defined by its dominant hot and humid climate, the predominance of a forest physiognomy, its geographic continuity, its peri-equatorial condition and the nature of its immeasurable socio-biodiversity30. Part of Brazil’s national heritage, the Biome is part of the Amazon Biogeographic Domain, which covers almost seven million km2 and is shared by nine countries: Bolivia, Brazil, Colombia, Ecuador, Guyana, French Guiana, Peru, Suriname and Venezuela; with more than 60% (4.212.923 km²) in Brazil31.
The diversity and complexity of the Amazon Biome form part of the ecological processes of nine types of forest cover and extensive contact areas (transition zones) with different habitats and variations in the composition and interaction of species, structure of natural communities and patterns of nutrient cycling. Because of its enormous extent of unbroken forests, the Amazon is very important for the stability of the regional climate. It moves large amounts of water vapour originating in the Atlantic Ocean and transports these across South America, ensuring the regulation of rainfall in places such as Argentina, Paraguay and south-central Brazil. It is estimated that the evaporation and transpiration of Amazonian vegetation, composed of trees up to 50 meters high, is about seven trillion tons of water per year pumped into the atmosphere32. We now know that Amazonian populations played an extremely important role in the formation of landscapes and the models of current landscapes, influencing the abundance, richness and distribution of species, altering soil nutrients, as well as structures for water and fisheries management and for transport and communication networks, among other things.
According to the Ministry of the Environment MMA, 2,500 tree species (one-third of global tropical tree species) and 30,000 plant species (out of 100,000 in South America) are estimated to grow in the biome. Nevertheless, the level of knowledge of taxonomic groups is still considered unsatisfactory. Estimates make the region the largest reserve of tropical timber in the world. Its natural resources - which, in addition to wood, include huge stocks of rubber, nuts, fish and minerals, for example - represent an abundant source of natural wealth. The region also holds a great deal of cultural wealth, including traditional knowledge about the uses and ways of exploiting these natural resources without depleting them or destroying the natural habitat. All this greatness, however, does not hide the fragility of the local ecosystem. The forest lives by its own organic material and its delicate balance is extremely sensitive to any interference. The damage caused by anthropic (human) action is often irreversible. In addition, the natural wealth of the Amazon contrasts dramatically with low socio-economic regional indicators, low population density and increasing urbanization. The use of forest resources is therefore strategic for the development of the region.
The various Amazons
The Amazon Basin – The Amazon Basin is formed by the headwaters and tributaries of the Amazon River - the largest river in the world - and its entire drainage area, making it the most extensive hydrographic network on earth, occupying a total area of more than 6 million km², from its sources in the Peruvian Andes to its mouth in the Atlantic Ocean. This continental basin extends over several countries of South America: Brazil (63%), Peru (17%), Bolivia (11%), Colombia (5.8%), Ecuador (7%) and Guyana (0.2%). The average contribution of the volume of water in Brazilian territory is around 73% of the total33. The Amazon Basin corresponds to almost 40% of South America and 5% of the Earth's land area; it is the largest store of surface freshwater on the planet, with about 15% of the available total34.
The Amazon River Basin - a management area for implementing the National Water Resources Policy and the National Water Resources Management System, it is an artificially created territory35 in light of the need to establish an organizational basis for watershed management. It consists of the Brazilian portion of the Amazon river basin and the river basins on Marajó Island and in the State of Amapá, amounting to 3,870,000 km.
The Legal Amazon – The administrative unit known as the Brazilian Legal Amazon comprises the states of Acre, Amapá, Amazonas, Pará, Rondônia, Roraima, Tocantins, Mato Grosso and part of Maranhão, first established by Federal Law No. 5,173 (Article. 2)36, with subsequent amendments. Mato Grosso came to form part of this territory in the act of its creation, under Art. 45 of Supplementary Federal Law No. 3137 and the state of Tocantins, created by the Transitional Provisions of the Federal Constitution (Art. 13)38, in October 1988. Previously the limit of the Legal Amazon in this section was the 13ºS parallel, along which the State of Goiás was approximately divided into two, creating the state of Tocantins. The creation and administrative differentiation of this was related to the process of development, occupation and integration of this region into the networks and flows that guided development policy at the time, mainly through the granting of tax incentives for industry and policies of rural settlement.
Comprising an area of more than 5 million km² (two thirds of Brazil), the Legal Amazon encompasses the entire Amazonian biome, 37% of the Cerrado and 40% of the Pantanal39, and is characterized by a mosaic of ecosystems with significant differences both in terms of structure and interactions of communities and natural populations and occurrence and abundance of flora, fauna, fungi and microbiota species. Representing 59% of the Brazilian territory and 775 municipalities, the Legal Amazon brings together approximately 24.7 million inhabitants40, of which more than 433,000 are indigenous41 and members of several categories of traditional extractive communities such as rubber tappers, brazil nut collectors, artisanal fishers and babaçu coconut shellers, among others.
More than 350 communities of descendants o quilombos32 also live in the region. As well as the original indigenous populations, characterized by an intimate and specific relationship with the environment, and traditional communities who arrived in this region at different times and driven by different needs, nowadays there are other groups that have come more recently to occupy the region, with differing forms of production, from small rural producers to large agribusiness enterprises.
See data on conservation areas in this biome in our data panel
Caatinga
Nowadays the Serra is less rugged and impenetrable than in the time of my great-grandfather Dom João Ferreira-Quaderna. Nevertheless, access remains difficult and painful. It is covered with thorn trees interlaced with cat’s claw, touch-me-not, faveleira, xique-xique cactus, nettles, orchid trees and quince trees. Catolé palms and prickly cacti complete the vegetation, and it is said that the blood that soaked the earth and the stones during the reign of the Quadernas was so great that on Good Friday each year, the catolé palms would groan, the stones would glitter in the brown and the incrustations of silver or mica, and the crowns of the Turk’s cap cacti monks begin to seep blood, red and alive as if it had just been spilt. - Ariano Suassuna.42
The Caatinga biome is exclusive to Brazil and a unique biological heritage of the planet, located in the semi-arid region (comprising the agreste and the sertão) and covering almost all the Northeast (except the coastal zone and the west of Maranhão, Piauí and Bahia states) and some areas in the north of Minas Gerais. Its area is approximately 84 million hectares (844,453 km²), equivalent to 11% of the national territory43.
The word "Caatinga" is of tupi-guarani origin and means a whitened forest, a feature that is clear during periods of drought, when the vegetation sheds its leaves and the trunks and branches turn pale44. The special characteristics of their biological communities are many, due to variations of environments and microclimates and to the different survival strategies developed by the plant species during the long dry periods. Among the strategies is the loss of foliage, as a way to avoid losing water; the foliage is quickly renewed following renewed rainfall.
The climate of the caatinga presents extreme characteristics, from the highest annual average temperature to the lowest levels of humidity and rainfall. Rainfall is restricted to a few months of the year, with critical episodes of lack of rainfall occurring over successive years. In the agreste region, the hinterland between the coastal range and the drier interior, the rains are more abundant. Climatic and edaphic diversity favours physiognomic diversity, such as dry upland forests and shrub and herbaceous plant communities, cacti and bromeliads, and many endemics plants and animals. One example is Lear's Macaw (Anodorhynchus leari), one of the caatinga's most endangered and endemic species 45.
By 2017, more than 60% of the Northeast population lived in the caatinga (27 million people, about 18% of Brazil's total population), despite being one of the regions with the lowest human development indicators in the country43 and 44. The biodiversity of the caatinga supports a number of agropastoral economic activities and chemical, pharmaceutical, food and cosmetic industries. An example is the carnauba tree (Copernicia prunifera), a native palm (leaves, stem, stalk, fibre, fruit and roots) widely exploited in the manufacture of handicraft and industrial products, such as carnauba wax used for preserving food products, as floor covering and for the protection of motor vehicles.
Despite its importance, the caatinga is a biome little studied in academic circles and one of the most threatened. It is estimated that each year the caatinga loses an area of native vegetation equivalent to twice the city of São Paulo43 and 44. In recent years, deforestation has grown at an increasing rate, mainly through illegal logging, resulting in the loss of half of this biome.
See data on conservation areas in this biome in our data panel
Learn more about the traditional populations of the Caatinga and Cerrado at - http://www.cerratinga.org.br/populacoes/
Cerrado
I am the hardness of these hill
covered,
flowered,
splintered by the axe,
ripped open, lacerated.
Burned by fire
Pulped
Scorched
and reborn - Cora Coralina46
The Cerrado is the second largest biome in Brazil and covers 2,036,448 km², or 204 million hectares (22% of the national territory), extending its domain through the states of Goiás, Tocantins, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Bahia, Maranhão, Piauí, Rondônia, Paraná, São Paulo and the Federal District, as well as parts of Amapá, Roraima and Amazonas. However, a large part of this biome is located in the Brazilian Central Plateau, the place of origin of the great hydrographic basins of Brazil and of the South American continent, as well as being a watershed in the country47. Many rivers that flow into the Araguaia-Tocantins, Paraná, São Francisco and even the Amazon basins, are born in the Cerrado, which explains the existence of numerous springs, lakes and rivers of various orders. Because of this role, the Cerrado is critical in water security for many regions of the country, and such is recognized as the "cradle of Brazilian waters".48
Like the other biomes, the Cerrado, "is not a single biome, but a complex of biomes, formed by a mosaic of communities belonging to a gradient of ecologically related formations, ranging from open grasslands to dense savanna."49
These characteristics "confer a peculiar structure and functionality (...) and include another element of importance in the determination of certain terrestrial environments"48, the natural occurrence and recurrence of fire. This characteristic has been demonstrated through several studies that attest the presence of fire for millions of years, as a determining factor in the physical characteristics of the Cerrado.
All these aspects contribute to the great richness and high biological diversity and endemism, which raise the Cerrado to the category of a global hotspots. There are more than 11,000 recorded species of native plants. It is a refuge for 13% of butterflies, 35% of bees and 23% of termites in tropical areas. This diversity is favoured by the high productivity of the Cerrado, resulting from its tropical climate strongly marked by well-defined dry (April-May to September-October) and wet (September-October to March-April) seasons. The minimum temperature reaches 10°C or less in winter, and up to 40°C in summer, but is generally predominantly high throughout the year47,48,49 and 50.
See data on conservation areas in this biome in our data panel
Alternative Cerrado management techniques are already practiced, given the diversity of native fruits widely consumed and traded in urban centres, such as pequi (Caryocar brasiliense), buriti(Mauritia flexuosa), mangaba (Hancornia speciosa), cagaita (Eugenia dysenterica), bacupari (Salacia crassifolia), cerrado cashew (Anacardium humile), araticum (Annona crassifolia) and barú nuts (Dipteryx alata).
" Baru is a fruit typical of the Brazilian Cerrado. The nut is about 23% protein and is a foodstuff with high nutritional value. "In recipes and confections baru nuts can substitute peanuts, cashews or any other nuts. It is also used in various recipes, sweet or savoury, such as cakes, breads, nut brittle, cookies, brigadeiro, cereal bars, granolas and paçoca".51
Intense deforestation and predatory extraction in the Cerrado have led to the disappearance and loss of such species as the Baru, whose wood is prized for its durability and quality, with fungicidal properties51. The nut inside its fruit, protected by a hard shell, has a flavour similar to the peanut, high nutritional value, and is appreciated and traded worldwide.
For thousands of years, the Cerrado has been managed by the different indigenous human populations, and also by quilombolas, geraizeiros, riverine dwellers, babaçu nut collectors, vazanteiros (or "barranqueiros"), families that inhabit the islands and banks of rivers such as the São Francisco, Tocantins and Araguaia and whose agriculture is associated with the cycles of flood, full, ebb and drought51 and 52. Knowledge of fruits, woods and numerous herbs that these populations hold, and their understanding of the biome are an important feature of Brazil’s historical and cultural heritage. According to the Ministry of the Environment50, more than 220 Cerrado species have medicinal uses, and more than 416 can be used to restore degraded soils, as wind barriers, protection against erosion, or to create habitats for natural pest predators.
Geraizeiros
"Along the banks of the São Francisco River, where its waters cross the north of Minas Gerais and in the transition area between the Cerrado and the Caatinga, in the west of Bahia, live the geraizeiros, recognized as farmers of the high plains, scarps and valleys of the Cerrado The name for these populations derives from the term "Gerais" as a synonym for the Cerrado. According to its oldest inhabitants, the term Cerrado was not used, only the Gearis, hence the name geraizeiros.51
Atlantic forest
Passaredo
Hey, hooded siskin
Hi, linnet
Blackbird, musician wren
Hi, whistling duck
Nightjar
Finch, tinamou
Flee, picazuro pigeon
Come on, plumbeous seedeater
Song thrush, wood ibis, parrotlet
Flee, crimson tanager
Flee, masked tanager
Flee, nightingale, striped cuckoo
Disappear, double-collared seedeater
Move, corn bunting
Hide, hummingbird
Fly, solitary tinamou
Fly, long-tailed tyrant
Quiriquiri falcon
Rufous-bellied thrush
Take care
QThe men are coming
Men are coming
Men are coming - Chico Buarque / FH53
The Atlantic Forest was the first and most intensely exploited biome since the colonial period. Originally, the Mata Atlântica covered a strip along the Brazilian coast, in 17 Brazilian states from Rio Grande do Norte to Rio Grande do Sul of approximately 1,300,000 km² 54; in the southeast, this biome extended 300-400 km inland, due to climatic conditions and relief strongly marked by the mountain slopes that accompany the Serra do Mar, constituting new physiognomies from the coastal plain to the seasonal forests of the high plateau55 and 56. Using fire to clear the first pastures, shifting cultivation and coffee growing, European settlers gradually took control of their lands and felled the forest. Even now, the remnants of this forest remnants continue to be drastically reduced. In the period 2015-2016, for example, there was a bigger increase in deforestation in this biome, in states such as Bahia and Minas Gerais; in the latter, with the conversion of forest for charcoal burning and eucalyptus plantations55 and 57.
The Atlantic Forest represents a mosaic of ecosystems characterized by the Dense Ombrophilous Forest, Open Ombrophilous Forest and Mixed Ombrophilous Forest; by Deciduous and Semi-deciduous Seasonal Forest, and other associated ecosystems such as mangroves, restingas (moist broadleaf forest on sandy soils), high altitude grasslands, inland swamps and oceanic islands. Despite representing only 0.8% of the earth's surface and having been heavily impacted, it is estimated that about 20,000 plant species (about 35% of Brazilian species) and 2,000 species of vertebrate animals survive in the Atlantic Forest, not counting insects and other invertebrates55. As a consequence, this biome has been designated a Biosphere Reserve by UNESCO and part of Brazil’s National Heritage by the Federal Constitution. In addition, it comes under the protection of Federal Law No. 11.428 / 2006, which deals with the use and protection of the native vegetation of the Atlantic Forest Biome, as well as Decree No. 6,660 / 2008 which regulates this law. Known as the Law of the Atlantic Forest, it also regulates the sustainable exploitation of its resources by defining different parameters for each stage of the remaining native vegetation - primary, initial secondary, mid and advanced regeneration58.
See data on conservation areas in this biome in our data panel
This region concentrates approximately 72% of the Brazilian population (approximately 145 million inhabitants) in 3,429 municipalities, responsible for 70% of the Gross National Product55 and 57. There are thus several challenges for the conservation of the Atlantic Forest and, at the same time, to the search for alternative economic solutions that reduce the pressure on this ecosystem. Among these challenges are maintaining primary vegetation, preserving springs and water sources – many of these that supply the cities are found in densely consolidated areas - and controlling collection of its natural resources threatened with extinction, such as orchids, ferns and the juçara palm heart (Euterpe edulis), and hunting of wild animals. Another challenge is to respect the ways of life and the granting of control over their traditional territories to the different traditional populations - caiçaras, caipiras, açorianos, quilombolas, or artisanal fishers59 e 60, who complement the indigenous sociocultural diversity, represented by groups such as the: Guarani, Kaingang, Xocleng, Pataxó, Tupiniquim, Krenak and Terena (see other groups here PIB. By resisting in their territories, these traditional peoples contribute to conservation of extensive forest fragments and to maintenance of ecosystem services, carbon stock and model practices for sustainable management, reconciling preservation of fauna and flora with maintenance of their customs, knowledge and practices.
Pampa
For as far as the eye can see across the horizon there is the pampa. An immense green sea that has its edges along the River Plate and its end, if it has an end, in Patagonia, much further south. When they realised its lonely enormity (...), the Argentinians called it a "desert." - Voltaire Schilling*61
The Pampas or southern grasslands almost always bring to mind a landscape dominated by low relief, in a cold and arid climate and at the same time hot and humid, basically covered by grasses and sparse trees. Although these elements are a part, none of them properly and fully describe the landscapes and riches of this biome. In one sense, the lack of a consensus around the various names equally used - such as Campos do Sul, Campanha Gaúcha, Pampas Gaúcho, or simply Campos - or around the cultural origin and identity of the gaúcho people, reflects the importance of the Pampas in academic debates and public policies concerning its conservation. Like other biomes, their views cannot be separated from economic and cultural history 62. The first European settlers found in this region of South America grassy landscapes, open and very suitable for the economic activities that were subsequently implanted, such as ranching62.
The Pampas cover parts of Brazil, Uruguay, Paraguay and Argentina (about 750,000 km²), where 35 million people live. They represent 2.0% of the Brazilian territory (18 million hectares or 176,496 km²), the second smallest of the biomes in size. Globally, they account for 2.5% of the world's63 and 66 farm land. They are considered natural ecosystems, in which the predominance of grasses intermixed with tree-sized species have lived through different climatic changes, across geological time, as well as through human influence with the use of fire.
Thousands of years ago, the climate was drier and colder, which allowed the formation of prairies and then, only four thousand years ago, a wetter climate favoured the expansion of araucarias and forests along the waterways and in the mountains66. The seasons are well defined, the rains spread throughout the year and the average temperatures vary from 15ºC to 18ºC. The Pampas represent an immense natural, cultural and associated material heritage. Because of their fertile soils and the presence of grasses, the Pampas supported extensive livestock ranching for over three hundred years, when domesticated cattle were introduced by the Jesuits into their Rio Grande do Sul missions in the 17th century62. Since then, other economic activities have contributed to the reduction of biodiversity, especially in the last twenty years, when for example, Rio Grande do Sul moved from about 63% coverage by the Pampas to currently, over 40% of the biome destroyed63. The introduction of large-scale agriculture (soybean and rice), exotic species such as the African grass Eragrostis plana, nd woody species such as Pinus sp and Eucalyptus, have greatly increased fragmentation of the remaining areas66.
The current Pampas conservation policies are still insufficient. The remaining areas are in danger of being further reduced with the approval of Rio Grande do Sul State Law No. 145 of November 29, 2016, which allows increased tree plantations64 and 66.
See data on conservation areas in this biome in our data panel.
There are excellent initiatives for Pampa conservation, such as the submission of a proposal by environmentalists in Rio Grande do Sul for a parliamentary amendment to include the Southern Grasslands in the list of biomes constituting Brazil’s national heritage, under paragraph 4 of article 225 of the Federal Constitution65. There are also projects to restore areas degraded by erosion in agropastoral areas and measures to create ecological corridors, integrating conservation and regional development. Other growing alternative uses are the cultivation of pecans (Carya illinoensis) and olives (Olea europaea L.), both of which have great economic potential67. The Pampa is a source of genetic variability for several species that underpin our food chain. Institutions and research centres in Rio Grande do Sul have also devoted themselves to collecting seed varieties of corn, rice and beans, products of family farming systems over hundreds of generations. With this collection, researchers have sought details of morphological characteristics, the history of the variety, its resistance to diseases, climate suitability, among other aspects. These landraces, such as Azorean white maize, are multiplied and distributed to family farmers68.
Pantanal
The river that described a curve behind our house was the image of
a soft glass described a curve behind the house.
Then a man passed by and said: That curve the river makes for
behind your house is called an enseada.
It was no longer the image of a glass snake turning a corner
behind the house.
It was an “enseada”.
I think the name impoverished the image. - Manoel de Barros (1916-2014)69
The Pantanal biome, covering an area of approximately 250,000 km², is located between southern Mato Grosso and north-western Mato Grosso do Sul in Brazil and northern Paraguay and eastern Bolivia, where it is known as the Chaco. It is the largest wetland in the world, although it is the smallest biome by size in Brazil: about 150,000 km² (1.8% of the national territory). Although the landscape is identified by its "savanna" characteristics, the Pantanal is an important natural connection between the River Plate and Amazon basins, strongly influenced by this and by the Cerrado and Atlantic Forest biomes.
Thus, the Pantanal shares common species with each of these biomes70, 71, 72 and 73 and is a refuge for various species in danger of extinction, but which here encounter favourable living conditions, such as the bird that is the symbol of the Pantanal – the tuiuiú73. More than two thousand species of plants have already been identified in this biome, in addition to its immense pharmaceutical potential 73. The Pantanal is recognized as part of the National Heritage by the Brazilian Federal Constitution and a Biosphere Reserve and World Natural Heritage site by UNESCO. It also hosts three Ramsar Sites (Wetlands of International Importance): the Mato Grosso Pantanal National Park, the SESC Pantanal Private Natural Heritage Reserve and the Fazenda Rio Negro Private Natural Heritage Reserve73.
Its rivers rise in the high plains, from where they run great distances, winding along their beds towards the lowlands72. One of the most important is the Cuiabá River, historically known for its economic importance to the state capital of the same name, in the state of Mato Grosso. It served as a transport route and production outlet between the eighteenth and mid-twentieth centuries. Many families still live along the river. One of the landscapes seen when sailing along its plain is the Serra do Amolar, near the border with Bolivia, between the municipalities of Cáceres (MT) and Corumbá (MS).
In the Pantanal, the different ecosystems and the economic activities of its inhabitants are defined by the dynamics of flooding and, influenced by the strongly seasonal climate, the cycle of high and low waters drastically changes the landscape of this biome. This area of seasonally flooded lowland accounts for 50% of the Upper Paraguay River basin, which supplies a population estimated at more than a million inhabitants in Brazil, and a significant portion of the inhabitants of Bolivia and Paraguay71 and 72. The use and management in the lowland reside in ranching, fishing, tourism and, to a lesser extent, agriculture. On the high plains, where its headwaters are located, cereal and livestock farming dominate, with a significant impact on Pantanal environments, silting up and polluting rivers, aggravated by the large-scale use of biocides71.
Cultural diversity reveals further riches in the Pantanal, present in traditional communities, such as the Guató indigenous People, os quilombolas, bait collectors, riverbank dwellers and artisanal fishers, as well as cattle ranchers, known for the characteristic rhythm with which they cross the flooded lowlands with their herds73. This cultural diversity has greatly influenced the formation of Pantanal culture.
There has been much discussion among scientists and staff of public agencies concerning alternative economic activities that can benefit from the Pantanal's natural wealth and generate income for rural family agriculture communities. One such alternative has been the development of beekeeping, which can occur on a large scale due to the existence of natural areas, and with low environmental disturbance.
Agrobiodiversity
Author: Juliana Santilli (Founding Member of ISA) (2010)
What is agrobiodiversity?
The concept of agrobiodiversity reflects the dynamics and complex relationships between human societies, cultivated plants and the environments in which they coexist, with implications for policies for conservation of cultivated ecosystems, promotion of food and nutritional security of human populations, social inclusion and sustainable local development.
Biodiversityb or biological diversity – the diversity of life forms - covers three levels of variability: species diversity, genetic diversity (the variability within the set of individuals of the same species) and ecological diversity, which refers to different ecosystems and landscapes. This also applies to agrobiodiversity, which includes species diversity (e.g. different species of cultivated plants, such as corn, rice, pumpkin, tomato etc.), genetic diversity (e.g. differing varieties of maize, beans, etc.) and the diversity of agricultural or cultivated ecosystems (for example, traditional farming and fallow farming systems, also known as coivara or itinerant systems, agroforestry systems, terraced crops and planting on flooded land etc.). Agroecosystems are areas of natural landscape transformed by humans for the purpose of producing food, fibre and other raw materials 74. One of the characteristics of agroecosystems is the predominance of species of interest to humans and a spatial organization that structures and facilitates the work of production, according to Katia Marzall75.
Agrobiodiversity, or agricultural diversity, constitutes an important part of biodiversity and encompasses all those elements that interact in agricultural production: areas cultivated or used for domestic animals, directly or indirectly managed species such as those grown and their wild relatives, weeds, pests, pests, pollinators, predators, symbionts76 (organisms that are symbiotic, in other words that live alongside others) and the genetic diversity associated with these (also called intraspecific diversity, that is, within the same species). Diversity of species is called interspecific diversity.
The Convention on Biological Diversity does not provide a definition of agrobiodiversity but, according to its Decision V/5, agrobiodiversity is a broad term that includes all the components of biodiversity that are relevant to agriculture and food, and all the components of biodiversity that constitute agroecosystems: the variety and variability of animals, plants and micro-organisms at the genetic, species and ecosystem levels necessary to sustain the key functions of agroecosystems, their structures and processes. Therefore, the components of agricultural biodiversity include: plant diversity, both domesticated and wild (some authors exclude wild plants and animals from the definition of agricultural biodiversity on the grounds that, although they are important for farmers, they are not part of agricultural systems77); the diversity of domestic animals (of about 50,000 species of mammals and birds known, approximately forty have been domesticated and, of these, farmers have developed about 5,000 breeds adapted to local environmental conditions and to specific needs); the diversity of aquatic fauna (fish and other aquatic species are an integral part of many important farming systems); belowground diversity (roots carry nutrients and water to plants and stabilize the soil); microbial diversity (micro-organisms recycle and provide many nutrients needed by plants, among other functions); the diversity of insects (such as bees and other pollinators), spiders and other arthropods (grasshoppers, centipedes, and others) who often act as natural enemies of beings harmful to plants; the diversity of ecosystems78. In this text we will focus mainly on the diversity of cultivated plants and agroecosystems, rather than on the diversity of domestic animals and other components of agricultural biodiversity.
Although the terms "agricultural biodiversity" and "agrobiodiversity" are often used interchangeably, there are authors79 who argue that agricultural biodiversity and agrobiodiversity have different meanings. "Agrobiodiversity", an older and more common term, is taken to define the biological diversity of cultivated ecosystems. "Agrodiversity" would be a broader term used to refer to "the many ways in which farmers use the natural diversity of the environment for agricultural production, including not only selection of species and varieties of plants for cultivation, but also the management of lands, waters, and of biota as a whole80”.
Another definition of "agrobiodiversity" would be "the variety resulting from the interaction between the factors that determine the agroecosystems: the plant genetic resources, biotic and abiotic environments and management practices81. We will use the term "agrobiodiversity" because it is better known.
Agrobiodiversity is essentially a product of human intervention over ecosystems: of human inventiveness and creativity in interacting with the natural environment. Cultural processes, knowledge, practices and agricultural innovations, developed and shared by farmers, are a key component of agrobiodiversity. The practices of management, cultivation and species selection, developed by farmers over the last 10,000 to 12,000 years, are largely responsible for the enormous diversity of cultivated plants and agroecosystems and, therefore, we cannot consider agrobiodiversity disassociated from the contexts, processes and cultural and socioeconomic practices that determine and condition it. Therefore, in addition to biological, genetic and ecological diversity, there are authors who add a fourth level of variability: the socioeconomic and cultural systems that generate and construct agricultural diversity.
For Harold Brookfield, agricultural diversity includes the diversity of land ownership systems used for agriculture, differences between farmers in terms of access to land, spatial distribution and size of farms, divisions of labour by age, gender and labour cooperation, the dependence of farmers on jobs external to rural property, among others. Brookfield emphasizes that no agricultural system can be understood without considering the ways in which rural properties organize themselves and how forces (social, economic and political) interact to influence and shape such organization. He highlights the crucial importance of the dynamism of agrobiodiversity, a "constantly changing quilt created by the relationships between people, plants and the environment, that are always dealing with new problems and in search of new paths". According to Brookfield, the "adaptive dynamism" of agrobiodiversity is the most important characteristic for its survival and for the recovery of what has already been lost. After all, farmers have the ability to adapt to both adversity and opportunity, and processes of learning and experimentation are constantly being renewed82.
Diversity results from natural as much as cultural factors. Thus, there are societies that adapt rice varieties to aquatic cultivation, submersed in water, in humid regions, and there are others that adapt rice varieties to cultivation in dry regions. Different varieties of corn can be used to eat directly from the ear, to feed animals, to make popcorn and flour or to brew beer. They are also used for ornamental (especially those with coloured pigments), medicinal or religious purposes. Agronomist Jack Harlan says he was watching an Ethiopian farmer select (for planting the following year) the seeds of sorghum varieties with crooked ears. When he inquired as to the reason for this choice, the Ethiopian farmer replied simply: "Because these are easier to hang from the roof"83.
Other farmers have selected sweet sorghum varieties for chewing. Other varieties of sorghum have been separated to make bread and beer, and varieties with more resistant fibres to make baskets and to use for building. The same species may be used for food or medicine, and different parts of the same plant may also have different uses. Plants also have uses in rituals and religious ceremonies, and many names can be given to varieties of the same species. Agricultural diversity can also be expressed in characteristics that are perceptible to the human eye, such as variations of colour, shape, height, size and shape of leaves, genetic variations, resistance to droughts, pests and diseases, high nutritional content, etc., and their loss is difficult to accurately assess or measure. The extinction of agricultural knowledge, practices and knowledge is even more difficult to assess and measure.
Even if the extent of the loss cannot be estimated accurately, agricultural diversity is threatened, and it forms the basis for the survival of rural populations, especially low income populations. The Report on the State of the World’s Plant Genetic Resources presented at the 4th International Technical Conference on Plant Genetic Resources held in Leipzig, Germany, from June 17 to 23, 1996, was an important warning about the serious genetic and cultural erosion brought about by modern farming systems. The report84 was the first systematic global assessment of the status of conservation and use of plant genetic resources on the planet. According to the report, over the last hundred years, farmers have lost between 90% and 95% of their agricultural varieties. The report also states thaT:
- In South Korea, only a quarter of the fourteen native plant varieties grown in gardens and vegetable gardens in 1985 continued to exist in 1993. Only 20 percent of the maize varieties that existed in Mexico in the 1930s still exist today.
- In the United States, 95% of cabbage varieties, 94% of pea varieties, and 81% of tomato varieties have ceased to exist over the last century. Of the 7,098 apple varieties existing between 1804 and 1904, 86% no longer exist.
- In China, of the 10,000 varieties of wheat used in 1949 only a thousand were still used in the 1970s. Until the 1970s, about 5,000 varieties of rice were grown in India, of which only five hundred still exist, and between ten and twenty varieties occupy most of the Indian territory.
The loss of agricultural biodiversity is mainly caused by the substitution of local and traditional varieties, characterized by their high genetic variability, by "modern" varieties, with high yields and a restricted genetic base. According to the report, this is the main cause of genetic erosion (cited in 81% of national reports submitted by countries). Both species and cultivated varieties of these species have disappeared, and not just species domesticated by humans, but their wild relatives as well continue to disappear, due to the rapid devastation of natural ecosystems.
In some cases, the disappearance of a variety may not necessarily lead to the loss of genetic diversity, since its genes may also exist in other varieties; but the varieties themselves represent a unique combination of genes, with values and uses also unique. It is also estimated that the loss of a plant can cause the disappearance of forty types of animals and insects, which depend on it to survive, as well as in addition to genetic and molecular combinations unique in nature.85
The loss of diversity of domestic animal breeds is also extremely worrying if we consider their many uses for humans: they provide food (meat, eggs, milk, cheeses etc.), clothing (cotton, wool, fur etc.), transport, etc. Animals are also used in sporting practices and as guinea pigs for scientific experiments, in religious rituals, as food for other animals (baits for fishing, for example) etc. As with plants, the use of animals varies in different cultures. In countries such as China, Vietnam and Korea, for example, dog meat is used for human consumption (there is even a belief that it improves sexual performance). In China, there are five species of turtle that are raised on farms and sold in regional markets for human consumption and medicinal uses. In Singapore, the fried black scorpion is considered a tasty treat (the high temperatures in which it is prepared neutralizes its venom); kangaroo meat is served even in pizzerias in Australia and ants (mainly içá and saúva), taquara larvae and hornets are part of the diet of some indigenous Amazonian peoples. In Argentina a barbecue of bull testicles is eaten; in Bolivia, llama meat is a typical dish; and it is common to find roasted guinea-pigs in restaurants in Bolivia and Colombia.
According to the Report on the State of the World's Animal Genetic Resources for Food and Agriculture, about 20% of the world's breeds of cows, goats, pigs, horses and birds are threatened with extinction, and in the last six years 62 breeds of animals became extinct, representing the loss of almost one breed per month. This report was released during the 1st International Technical Conference on Animal Genetic Resources for Food and Agriculture held in Interlaken, Switzerland, from December 3 to 7, 200786.
There are also estimates that, over the course of the last century, of the 3,831 breeds of cattle, buffalo, goats, pigs, sheep, horses and donkeys in existence, 16% became extinct and 15% became rare, and 617 breeds of domestic animals have disappeared since 1892 87. Both animal diversity and plant diversity are therefore under threat.
Notes and References
Saiba Mais
- Biodiversidade nas Unidades de Conservação apoiadas pelo Programa Áreas Protegidas da Amazônia (ARPA).
- CICES (The Common International Classification of Ecosystem Services)
- Costanza, R.; De Groot, R.; Sutton, P.; van der Ploeg, S; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. 2014. Changes in the global value of ecosystem services. Global Environmental Change, 26, 152-158.
- Síntese do Conhecimento Atual da Biodiversidade Brasileira. 2005. MMA, por Thomas Lewinsohn.
- Avaliação do Conhecimento da Biodiversidade Brasileira. 2005. (MMA), por Thomas Lewinsohn Vol. 1 e Vol. 2.
- Union for Ethical BioTrade 2010. Biodiversity Barometer 2010. 4p.
- Panaroma da Biodiversidade Global 3. Secretariado da Convenção sobre Diversidade Biológica. 2010. Brasília: MMA/Secretaria de Biodiversidade e Florestas, p. 94.
- Estrutura trófica e história natural das cavernas brasileiras - R.L. Ferreira e R.P. Martins.
- Matriz Brasileira de Serviços Ecossistêmicos: http://brazil.forest-trends.org/
- Portaria do MMA (358/2009) que institui o Programa Nacional de Conservação do Patrimônio Espeleológico.
- Instrução normativa do MMA (2/2009) para determinar metodologia classificatória do grau de relevância de cavidades naturais.
- Projeto de Lei 855/11 do Dep. Carlos Bezerra (PMDB-MT).
Notas e Referências
- WILSON, E.O.;PETER, F.A. 1988. Biodiversity. Washington, DC: National Academy Press. 521 p.
- LEWINSOHN, T. M. e PRADO, P. I. K. L.. "Síntese do Conhecimento Atual da Biodiversidade Brasileira'. In:LEWINSOHN, T. M. Avaliação do Conhecimento da Biodiversidade Brasileira. Ministério do Meio Ambiente – MMA, Brasília. 2006. Vol. 1. 269p.
- TAKACS, D. 1996. The idea of biodiversity. The John Hopkins University Press, Londres.
- NOSS, R. "Indicators for monitoring biodiversity: a hierarchical approach". Conservation Biology. Vol. 4 p 355 – 364. 1990.
- ASSESSMENT, Millenium Ecosystem. Dryland Systems. 2005.
- BPBES. Plataforma Brasileira de Biodiversidade e Serviços Ecossistêmicos. Sumário para tomadores de decisão: 1º diagnóstico brasileiro de biodiversidade e serviços ecossistêmicos / Plataforma Brasileira de Biodiversidade e Serviços Ecossistêmicos / Autoria e colaboração de Carlos A. Joly ...[et al.] – 1. ed. – Campinas, SP: Edição do autor, 2018.
- LEWINSOHN, T. M. e PRADO, P. I. K. L.. "Síntese do Conhecimento Atual da Biodiversidade Brasileira'. In:LEWINSOHN, T. M. Avaliação do Conhecimento da Biodiversidade Brasileira. Ministério do Meio Ambiente – MMA, Brasília. 2006. Vol. 1. 269p.
- LEWINSOHN, T. M. e PRADO, P. I. K. L.. "Quantas espécies há no Brasil?"'. Megadiversidade. V1, n1. Julho 2005.
- CHAPMAN, A. D. et al. Numbers of living species in Australia and the world. Edição 2. 2009.
- PIVELLO, Vânia Regina; METZGER, Jean Paul. Diagnóstico da pesquisa em ecologia de paisagens no Brasil (2000-2005). Biota Neotropica, v. 7, n. 3, 2007.
- WILCOVE, D.S., McLELLAN, C.H. & DOBSON, A.P. 1986. "Habitat fragmentation in the temperate zone". In: Soulé, M.S. (ed.) Conservation biology: the science of scarcity and diversity. 1986. Sunderland, Massachussets: Sinauer Associates.584p. 237-256.
- LOVEJOY, T.E., BIERREGAARD, R.O., RYLANDS, A.B., MALCOM, J.R., QUINTELA, C.E., HARPER, L.H., BROWN, K.S., POWELL, A.H., POWEL, G.V.N, SCHUBART, H.O.R & HAYS, M.B. 1986. "Edge and other effects of isolation on Amazon forest fragments". In: Soulé, M.E. (ed.). Conservation biology: the science of scarcity and diversity. 1986. Sunderland, Massachussets: Sinauer Associates.584p. 257-285.
- AIZEN, M.A. & FEINSINGER, P. 1994. "Forest fragmentation, pollination, and plant reproduction in a chaco dry forest, Argentina". ECOLOGY 75(2): 330-351.
- FORMAN, R.T.T. 1998. Land Mosaics: the ecology of landscapes and regions. Cambridge: Cambridge University Press. 632p.
- FORMAN, R. T. T. & GODRON, M. 1986. Landscape ecology. New York: John Wiley Press. 619p.
- TAYLOR, P.D. 1993. "Connectivity is a vital element of lanscape structure". OIKOS 68(3): 571-573.
- DIEGUES, Antonio Carlos. Aspectos Socioculturais e Políticos do Uso da Água. Disponível clicando aqui. Acesso em 17 de julho de 2017.
- CALLOU, Angelo Brás Fernandes. Povos do mar: herança sociocultural e perspectivas no Brasil. Ciência e Cultura, v.62, n.3. p.45-48, 2010.
- Ministério do Meio Ambiente (MMA). Biodiversidade Brasileira: Avaliação e identificação de áreas e ações prioritárias para a conservação, utilização sustentável e repartição dos benefícios da biodiversidade nos biomas brasileiros. Brasília: MMA/SBF, 2002, 404p.
- Ministério do Meio Ambiente (MMA). Zona Costeira e Marinha. Disponível clicando aqui. Acesso em 20 de junho de 2017.
- Secretariado da Convenção sobre Diversidade Biológica (2014) Panorama da Biodiversidade Global 4. Montréal, 155 p.
- Instituto Socioambiental (ISA). Zona Costeira. In: Campanilli, M.; Ricardo, B. Almanaque Brasil Socioambiental. São Paulo: Instituto Socioambiental, p. 195-241, 2007.
- Ministério do Meio Ambiente. 2010. Centro Nacional de Pesquisa e Conservação de Cavernas CECAV)/ Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Acesso em setembro de 2010. Disponível clicando aqui.
- CULVER, D. C. 1982. Cave life: Evolution and Ecology. Harvard University Press, Cambridge. 189 p.
- DECU V. 1986. "Some considerations on the bat guano synusia". Travaux de l’Institut de Spéologie Emile Racovitza 25: 41-51. e FERREIRA R.L. 1998. Ecologia de comunidades cavernícolas associadas a depósitos de guano de morcegos. Dissertação de Mestrado em Ecologia, Conservação e Manejo de Vida Silvestre da Universidade Federal de Minas Gerais, MG, Brazil, 85 pp.
- Sociedade Brasileira de Espeleologia. 2010. Acesso em dezembro de 2010. Disponível clicando aqui.
- COUTINHO, Leopoldo Magno. O conceito de bioma. Acta botanica brasílica, v. 20, n. 1, p. 13-23, 2006.
- Manual Técnico da Vegetação Brasileira. 2012. Instituto Brasileiro de Geografia e Estatística - IBGE. Ministério do Planejamento, Orçamento e Gestão. Disponível clicando aqui.
- MELLO, T. de. ABC da Floresta Amazônica - Filho da Floresta. Brasília: Conhecimento Editora, 2008.
- IBGE. 2004. Mapa de Biomas do Brasil – Primeira Aproximação.
- INSTITUTO SOCIOAMBIENTAL (ISA). Laboratório de Geoprocessamento com dados RAISG (Red Amazônica de Información Socioambiental Georreferenciada).
- PINTO, L.F. "Amazônia". 2007. In: Beto Ricardo e Maura Campanili (Ed.). Almanaque Brasil Socioambiental 2008. Instituto Socioambiental. P. 83-106.
- Agência Nacional de Águas.
- CARNEIRO FILHO, A., TOMASELLA, J., Trancoso, R. "Amazônia, desflorestamento e água". In Ciência Hoje. Vol. 40 no 239. Julho de 2007. Pg. 30-37.
- Resolução CNRH n° 32, de 15 de outubro de 2003.
- Lei Federal Nº 5.173, de 27 de outubro de 1966. Dispõe sobre o Plano de Valorização Econômica da Amazônia; extingue a Superintendência do Plano de Valorização Econômica da Amazônia (SPVEA), cria a Superintendência do Desenvolvimento da Amazônia (SUDAM), e dá outras providências. Senado Federal, Subsecretaria de Informações.
- Lei Federal Complementar nº 31 de 11 de outubro de 1977. Cria o Estado de Mato Grosso do Sul, e dá outras providências. Presidência da República, Subchefia para Assuntos Jurídicos - www.planalto.gov.br.
- Brasil. 1988. Ato das Disposições Constitucionais Transitórias: promulgada em 5 de outubro de 1988. Coletânea de Legislação Ambiental e Constituição Federal. Organização: Odete Medauar. 7ª ed. São Paulo: Editora Revista dos Tribunais. 2008. Coleção RT Mini Códigos. 1117p.
- INSTITUTO SOCIOAMBIENTAL (ISA). Laboratório de Geoprocessamento. 2009. Amazônia Brasileira 2009 (mapa). Edição especial Programa Áreas Protegidas da Amazônia (ARPA).
- INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA (IBGE). 2000. Censo de 2010.
- INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA (IBGE). 2010.
- SUASSUNA, Ariano. Romance d'a pedra do reino e o príncipe do sangue do vai-e-volta: romance armorial-popular brasileiro. Livraria J. Olympio, 1971.
- Leal, Inara R.; Tabarelli, Marcelo C.; Silva, José Maria C. Ecologia e Conservação da Caatinga. 2005. Recife: Ed.Universitária da UFPE, 2005, 822p.
- Ministério do Meio Ambiente (MMA) (2017).
- Fundação Biodiversitas (2017). Disponível clicando aqui.
- Cora Coralina. Poemas dos becos de Goiás e estórias mais. São Paulo: Global, 1993. p 47-49.
- Lima, Jorge E.F.Werneck (2011). Situação e perspectivas sobre as águas do Cerrado. Ciência e Cultura, São Paulo, v.63, n.3. Disponível clicando aqui. Acesso em julho de 2017.
- Fonseca, Claudia Padovesi (2005). Caracterização dos ecossistemas aquáticos dos Cerrados. In: Scarito, A.; Silva, J.C.S.; Felfili, J.M. (org.) Cerrado: Ecologia, Biodiversidade e Conservação. Brasília: Ministério do Meio Ambiente. 415-429 p.
- Coutinho, Leopoldo Magno (2006). O conceito de bioma. Acta Bot. Bras. v.20, n.1, p. 13-23. 2006.
- Ministério do Meio Ambiente (2017). O Bioma Cerrado. Disponível clicando aqui. Acesso em julho de 2017.
- Cerratinga. Acesso em julho de 2017.
- Quem são os vazanteiros. Cerradania. Acesso em julho de 2017.
- CHICO BUARQUE, de H. & HIME, F. Passaredo. Disponível clicando aqui. Acesso em janeiro de 2019.
- Instituto Brasileiro de Geografia e Estatística. 2017. Disponível clicando aqui. Acesso em novembro/2017.
- DEAN, Warren. A conservação das florestas no Sudeste do Brasil (1900-1955). Revista de História, n.133, p.103-115, 1995.
- Ministério do Meio Ambiente (MMA). Mata Atlântica Disponível clicando aqui. Acesso em 20 de julho de 2017.
- Fundação SOS Mata Atlântica. Atlas da Mata Atlântica. Disponível clicando aqui. Acesso em: julho de 2017.
- Brasil. 2006. Lei Federal Nº 11.428 de 22 de dezembro de 2006. Dispõe sobre a utilização e proteção da vegetação nativa do Bioma Mata Atlântica, e dá outras providências. Disponível clicando aqui. Acesso em novembro/2017.
- DIEGUES, Antonio Carlos. Aspectos Socioculturais e Políticos do Uso da Água. Disponível clicando aqui. Acesso em 17 de julho de 2017.
- Povos Indígenas no Brasil.
- Schilling, Voltaire. Histórias: o gaúcho Martín Fierro.
- Behling, H.; Jeske-Pieruschka, V.; Schüler, L.; Pillar, V. De P. 2009. Dinâmica dos campos no sul do Brasil durante o Quaternário Tardio. In: Valério De Patta Pillar; Sandra Cristina Müller; Zélia Maria de Souza Castilhos; Aino Victor Ávila Jacques. Campos Sulinos: conservação e uso sustentável da biodiversidade. Brasília: MMA, 13-24 p.
- Ministério do Meio Ambiente (MMA) (2017) Disponível clicando aqui. Acesso em 20 de junho de 2017.
- Foolman, F. M.; Silva, F. da; Losekann, M.B. A transformação do pampa: demandas e alternativas para a conservação. In: Wizniewsky, C. R. F.; Foleto, E. M. (org.). Olhares sobre o pampa [recurso eletrônico]: um território em disputa / organizadoras. Porto Alegre: Evangraf, 78-88 p.
- Wizniewsky, C. R. Fl.; Foletto, E. M. 2017. Políticas de conservação no pampa brasileiro. In: Wizniewsky, C. R. F.; Foleto, E. M. (org.). Olhares sobre o pampa [recurso eletrônico]: um território em disputa / organizadoras. Porto Alegre: Evangraf, 10-23 p.
- Achka, Marcel. 2017. El bioma pampa: un territorio en disputa. In: Carmen Rejane Flores Wizniewsky, Eliane Maria Foleto (org.). Olhares sobre o pampa [recurso eletrônico]: um território em disputa / organizadoras. – Porto Alegre: Evangraf, 125-139 p.
- Campos Sulinos. Revista Ecológico. 2017. Disponível clicando aqui. Acesso em 20 de julho de 2017.
- Bevilaqua, Gilberto A. P.; Anjos e Silva, S. D. dos; Antunes, I. F.; Barbieri, R. L.; Galho, A. M.; Bammann, Itanner. 2007. Banco de Sementes de variedades crioulas e tradicionais da agricultura familiar de clima temperado. Rev. Bras. Agroecologia, v.2, n.1, p. 654-657.
- BARROS, Manoel de. O livro das ignorãças. Rio de Janeiro: Record, 2001b. p 25.
- MITTERMIER, R.A.; MITTERMIER, C.G.; BROOKS, T.M.; P ILGRIM, J.D.; KPNSTANT, W.R.; FONSECA, G.A.B.; KORMOS, C. Wilderness and Biodiversity Conservation. Proceedings of the National Academy of Sciences, Cambridge, MA, v.100, p. 10309-10313, 2003.
- FARIA, Alcides; Nicola, Rafaela. Pantanal. In: Ricardo, B.; Campanilli, M. Almanaque Brasil Socioambiental. São Paulo: Instituto Socioambiental. p. 177-194, 2008.
- ZORZETTO, Ricardo. 2015. Rios com vontade própria. Revista FAPESP.. Acesso em julho de 2017.
- Ministério do Meio Ambiente (MMA). 2017. O Bioma Pantanal. Acesso em julho de 2017.
- CONWAY, G. “The properties of agroecosystems.” Agricultural Systems, Barking Essex: Elsevier, v. 24, nº 2, p. 95-117, 1987.
- MARZALL, K. “Fatores geradores da agrobiodiversidade – Influências socioculturais.” Revista Brasileira de Agroecologia, Porto Alegre: Associação Brasileira de Agroecologia, v. 2, n. 1, p. 237-240, fev. 2007b.
- QUALSET, C. O; McGUIRE, P.E. & WARBURTON, M.L. “Agrobiodiversity: key to agricultural productivity”. California Agriculture, Oakland: University of California, v. 49, p. 45-49, 1995.
- Wood, D.& LENNÉ, J. M. “Why agrodiversity?” In: wood, D. & Lenné, J.M (eds.). Agrobiodiversity: characterization, utilization and management. Wallingford, GB, Cabi Publishing, 1999. p. 1-13.
- CROMWELL, E. ; COOPER, D. & MULVANY, P.. “Defining agricultural biodiversity.” In: Centro Internacional de la Papa (CIP); Users´ Perspective With Agricultural Research and Development (UPWARD). Conservation and sustainable use of agricultural biodiversity: a sourcebook. 3 v. Manila: CIP-Upward, 2003. v. 1, cap. 1, p. 1-12.
- BROOKFIELD, H. & STOCKING, M .. “Agrodiversity: definition, description and design.” Global Environmental Change, Londres: Elsevier, v. 9, p. 77-80, 1999.
- BROOKFIELD, H. & PADOCH, C.. “Appreciating agrodiversity: a look at the dynamism and diversity of Indigenous farming practices.” Environment, Farmington Hills, MI: Gale Group, v. 36, p. 8-11, 1994.
- ALMEKINDERS, C. ; FRESCO, L. & STRUIK, P.. “The need to study and manage variation in agro-ecosystems.” Netherlands Journal of Agricultural Science, Den Haag: Royal Netherlands Society for Agricultural Ecosystems, v. 43, nº 2, p. 127-142, 1995.
- BROOKFIELD, H. 2001. Exploring agrodiversity. Nova York: Columbia University Press, . p. 21, 38, 41, 44 e 286.
- HARLAN, Jack R. The living fields: our agricultural heritage. Cambridge: Cambridge University Press, 1995, p. 164
- A elaboração do relatório envolveu 151 países, cerca de cinquenta organizações não governamentais, representantes do setor privado e especialistas. O relatório subsidiou a adoção da Declaração de Leipzig e do Plano Global de Ação para a Conservação e Utilização Sustentável dos Recursos Fitogenéticos para Alimentação e Agricultura. O Brasil concluiu a elaboração do 2º Relatório Nacional sobre a Situação dos Recursos Fitogenéticos para a Alimentação e Agricultura em dezembro de 2008 (CD distribuído pela Embrapa).
- KLOPPENBURG, J. & KLEINMAN, D.. “Plant germplasm controversy: analyzing empirically the distribution of the world´s plant genetic resources.” BioScience. Washington: American Institute of Biological Sciences, v. 37, nº 3, p.190-198, 1987.
- Consultar: International Technical Conference on AnimaL Genetic Resources for Food and Agriculture, 3-7 sept. 2007, Interlaken, Suiça. The State of the World´s Animal Genetic Resources for Food and Agriculture. Roma: FAO, 2007. Schiere, Hans. Perda da diversidade de espécies e de raças de animais domésticos: um tema quase esquecido. In: BOEF, Walter S. de et al (org.). Biodiversidade e agricultores: fortalecendo o manejo comunitário. Porto Alegre: L & PM, 2007. p. 53-59; EMBRAPA. Animais do descobrimento: raças domésticas da história do Brasil. 2ª ed. Brasília: Embrapa, 2006; Steane, D. “Biodiversity in domesticated animals.” In: WOOD, D. & LENNÉ, J. M (eds.). Agrobiodiversity: characterization, utilization and management. Wallingford, GB: Cabi Publishing, 1999. p. 59-85; Anderson, Simon; Centonze, Roberta. “Property rights and the management of animal genetic resources.” World Development, St. Louis, MO: Elsevier, v. 35, n. 9, p. 1529-1541, 2007; Ingrassia, Antonella; Manzella, Daniele & Martyniuk, Elzbieta. The legal framework for the management of animal genetic resources. Roma: FAO, 2005. (FAO Legislative Study, 89). Consultar também o v. 2, n. 4, de dez. 2005, da revista Agriculturas: experiências em Agroecologia, que trata de experiências de criação de pequenos animais e de sua importância para a produção de base familiar.
- THRUPP, L. A.. “The central role of agricultural biodiversity.” In: Centro Internacional de la Papa (CIP); Users’ Perspective With Agricultural Research and Development (UPWARD). Conservation and sustainable use of agricultural biodiversity: a sourcebook. 3 v. Manila: CIP-Upward, 2003. v. 1, cap. 3, p. 20-32.